Highly Diastereoselective Access to Densely Functionalized Piperidine Cores of Influenza Endonuclease Inhibitors via a Metal-Free S N 1 Approach.
Zhang, Y., Sun, C., Guo, L., Zhao, K., Bennett, F., Lam, Y.H., Gao, Q., Ruhl, K.E., Pirnot, M.T., Emmert, M.H., Hollenstein, K., Eddins, M.J., Su, H.P., Shao, G., Song, C., Lo, M.M., Peng, F., Qi, J., Crowley, B.M., McCauley, J.A., Price, I.R.(2025) J Org Chem 90: 1175-1179
- PubMed: 39745425
- DOI: https://doi.org/10.1021/acs.joc.4c02379
- Primary Citation of Related Structures:
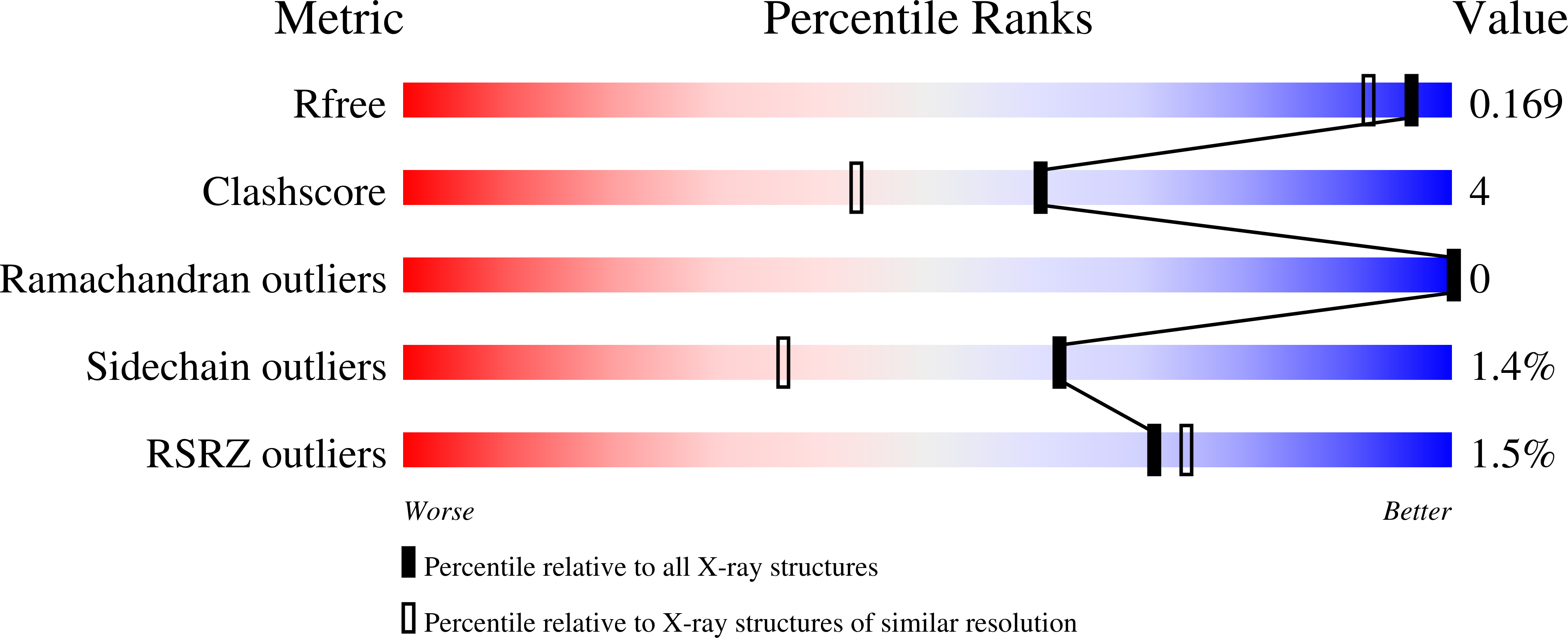
9DOJ - PubMed Abstract:
A novel, highly diastereoselective, and metal-free synthesis of multisubstituted piperidines via an S N 1 approach is reported in this study. The method allows for the preparation of highly functionalized compounds with exceptional diastereomeric selectivities and consistently reproducible yields. These compounds are of significant interest due to their remarkable biological activities toward influenza endonuclease.
Organizational Affiliation:
Discovery Chemistry, MRL, Merck & Co. Inc., 126 East Lincoln Avenue, Rahway, New Jersey 07065, United States.