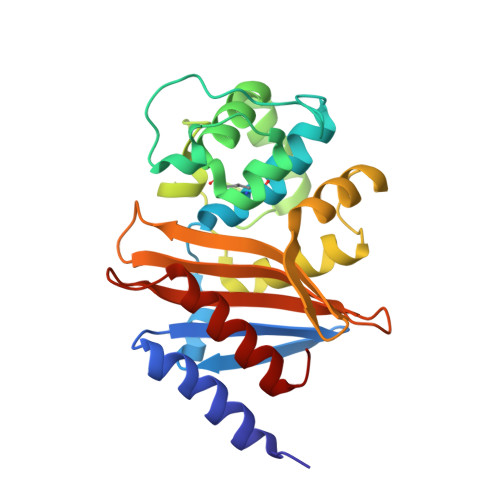
Design, synthesis, and crystal structures of 6-alkylidene-2'-substituted penicillanic acid sulfones as potent inhibitors of Acinetobacter baumannii OXA-24 carbapenemase.
Bou, G., Santillana, E., Sheri, A., Beceiro, A., Sampson, J.M., Kalp, M., Bethel, C.R., Distler, A.M., Drawz, S.M., Pagadala, S.R., van den Akker, F., Bonomo, R.A., Romero, A., Buynak, J.D.(2010) J Am Chem Soc 132: 13320-13331
- PubMed: 20822105
- DOI: https://doi.org/10.1021/ja104092z
- Primary Citation of Related Structures:
3FV7, 3FYZ, 3FZC, 3G4P, 3MBZ - PubMed Abstract:
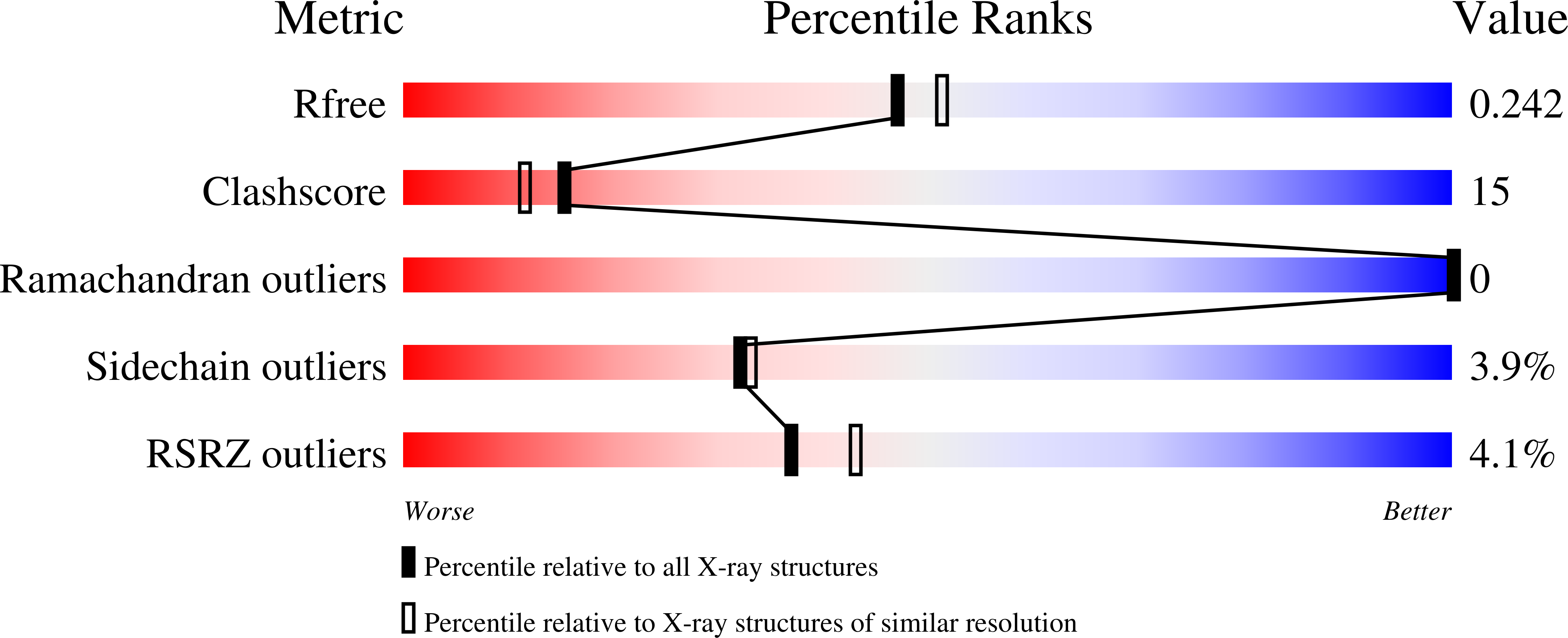
Class D ¦Â-lactamases represent a growing and diverse class of penicillin-inactivating enzymes that are usually resistant to commercial ¦Â-lactamase inhibitors. As many such enzymes are found in multi-drug resistant (MDR) Acinetobacter baumannii and Pseudomonas aeruginosa, novel ¦Â-lactamase inhibitors are urgently needed. Five unique 6-alkylidene-2'-substituted penicillanic acid sulfones (1-5) were synthesized and tested against OXA-24, a clinically important ¦Â-lactamase that inactivates carbapenems and is found in A. baumannii. Based upon the roles Tyr112 and Met223 play in the OXA-24 ¦Â-lactamase, we also engineered two variants (Tyr112Ala and Tyr112Ala,Met223Ala) to test the hypothesis that the hydrophobic tunnel formed by these residues influences inhibitor recognition. IC(50) values against OXA-24 and two OXA-24 ¦Â-lactamase variants ranged from 10 ¡À 1 (4 vs WT) to 338 ¡À 20 nM (5 vs Tyr112Ala, Met223Ala). Compound 4 possessed the lowest K(i) (500 ¡À 80 nM vs WT), and 1 possessed the highest inactivation efficiency (k(inact)/K(i) = 0.21 ¡À 0.02 ¦̀M(-1)? s(-1)). Electrospray ionization mass spectrometry revealed a single covalent adduct, suggesting the formation of an acyl-enzyme intermediate. X-ray structures of OXA-24 complexed to four inhibitors (2.0-2.6 ?) reveal the formation of stable bicyclic aromatic intermediates with their carbonyl oxygen in the oxyanion hole. These data provide the first structural evidence that 6-alkylidene-2'-substituted penicillin sulfones are effective mechanism-based inactivators of class D ¦Â-lactamases. Their unique chemistry makes them developmental candidates. Mechanisms for class D hydrolysis and inhibition are discussed, and a pathway for the evolution of the BlaR1 sensor of Staphylococcus aureus to the class D ¦Â-lactamases is proposed.
Organizational Affiliation:
Instituto de Investigaci¨®n Biom¨¦dica de A Coru?a (INIBIC), Complejo Hospitalario Universitario A Coru?a, 15006-A Coru?a, Spain.