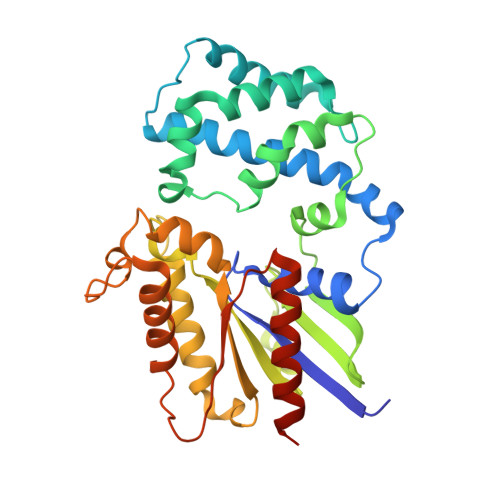
A P-loop Mutation in Galpha Subunits Prevents Transition to the Active State: Implications for G-protein Signaling in Fungal Pathogenesis
Bosch, D.E., Willard, F.S., Ramanujam, R., Kimple, A.J., Willard, M.D., Naqvi, N.I., Siderovski, D.P.(2012) PLoS Pathog 8: e1002553-e1002553
- PubMed: 22383884
- DOI: https://doi.org/10.1371/journal.ppat.1002553
- Primary Citation of Related Structures:
3QE0, 3QI2 - PubMed Abstract:
Heterotrimeric G-proteins are molecular switches integral to a panoply of different physiological responses that many organisms make to environmental cues. The switch from inactive to active G¦Á¦Â¦Ã heterotrimer relies on nucleotide cycling by the G¦Á subunit: exchange of GTP for GDP activates G¦Á, whereas its intrinsic enzymatic activity catalyzes GTP hydrolysis to GDP and inorganic phosphate, thereby reverting G¦Á to its inactive state. In several genetic studies of filamentous fungi, such as the rice blast fungus Magnaporthe oryzae, a G42R mutation in the phosphate-binding loop of G¦Á subunits is assumed to be GTPase-deficient and thus constitutively active. Here, we demonstrate that G¦Á(G42R) mutants are not GTPase deficient, but rather incapable of achieving the activated conformation. Two crystal structure models suggest that Arg-42 prevents a typical switch region conformational change upon G¦Á(i1)(G42R) binding to GDP¡¤AlF(4)(-) or GTP, but rotameric flexibility at this locus allows for unperturbed GTP hydrolysis. G¦Á(G42R) mutants do not engage the active state-selective peptide KB-1753 nor RGS domains with high affinity, but instead favor interaction with G¦Â¦Ã and GoLoco motifs in any nucleotide state. The corresponding G¦Á(q)(G48R) mutant is not constitutively active in cells and responds poorly to aluminum tetrafluoride activation. Comparative analyses of M. oryzae strains harboring either G42R or GTPase-deficient Q/L mutations in the G¦Á subunits MagA or MagB illustrate functional differences in environmental cue processing and intracellular signaling outcomes between these two G¦Á mutants, thus demonstrating the in vivo functional divergence of G42R and activating G-protein mutants.
Organizational Affiliation:
Department of Pharmacology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.