Crystallographic, kinetic, and calorimetric investigation of PKA interactions with L-type calcium channels and Rad GTPase.
Yoo, R., Haji-Ghassemi, O., Bader, M., Xu, J., McFarlane, C., Van Petegem, F.(2024) J Biological Chem 301: 108039-108039
- PubMed: 39615689
- DOI: https://doi.org/10.1016/j.jbc.2024.108039
- Primary Citation of Related Structures:
8UKN, 8UKO, 8UKP - PubMed Abstract:
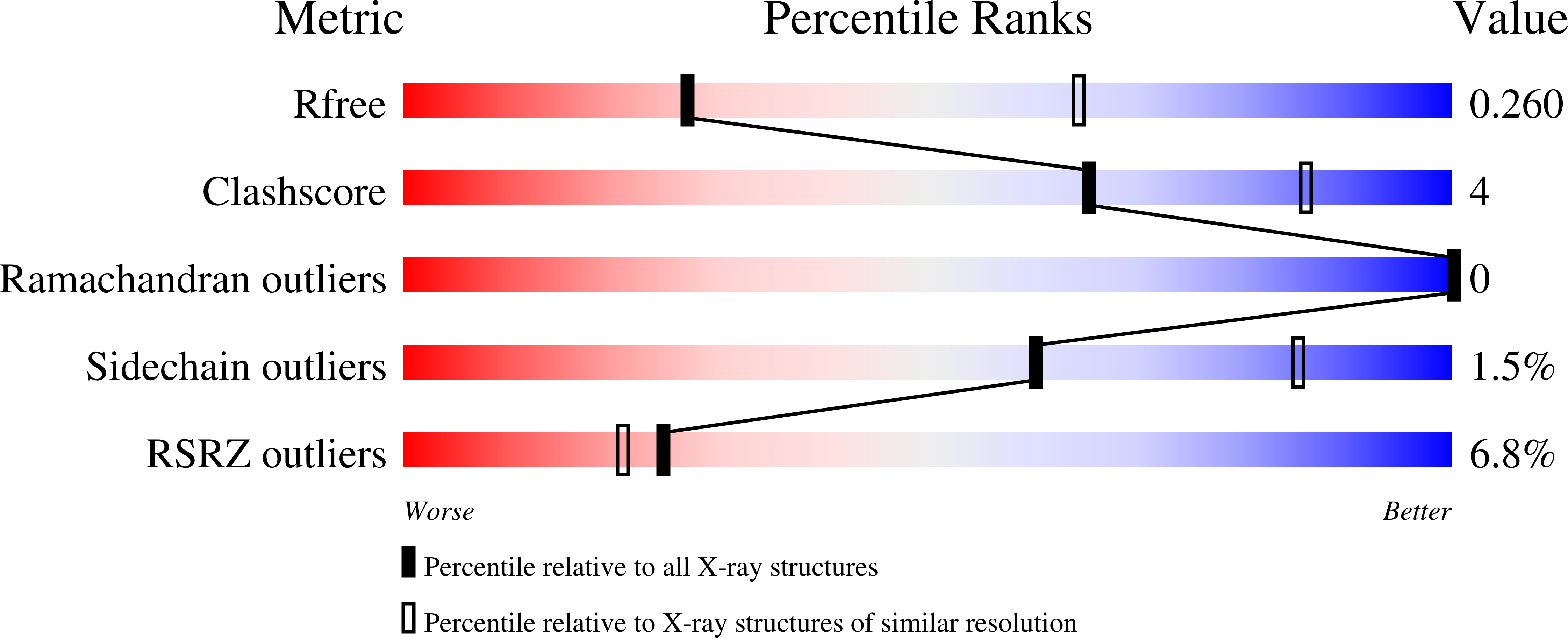
β-adrenergic signalling activates cAMP-dependent protein kinase (PKA), which regulates the activity of L-type voltage-gated calcium channels such as Ca V 1.2. Several PKA target sites in the C-terminal tail of Ca V 1.2 have been identified, and their phosphorylation has been suggested to increase currents in specific tissues or heterologous expression systems. However, augmentation of Ca V 1.2 currents in the heart is instead mediated by phosphorylation of Rad, a small GTPase that can inhibit Ca V 1.2. It is unclear how each of the proposed target sites in Ca V 1.2 and Rad rank towards their recognition by PKA, which could reveal a preferential phosphorylation. Here we used quantitative assays on three Ca V 1.2 and four Rad sites. Isothermal titration calorimetry (ITC) and enzyme kinetics show that there are two Tiers of targets, with Ca V 1.2 residue Ser1981 and Rad residues Ser25 and Ser272 forming Tier 1 substrates for PKA. These share a common feature with two Arginine residues at specific positions that can anchor the peptide into the substrate binding cleft of PKA. In contrast, PKA shows minimal activity for the other, Tier 2 substrates, characterized by low k cat values and undetectable binding via ITC. The existence of two Tiers suggests that PKA regulation of the Ca V 1.2 complex may occur in a graded fashion. We report crystal structures of the PKA catalytic subunit with and without a Ca V 1.2 and test the importance of several anchoring residues via mutagenesis. Different target sites utilize different anchors, highlighting the plasticity of PKAc to recognize substrates.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of British Columbia, Life Sciences Institute, 2350 Health Science Mall, Vancouver, British Columbia V6T 1Z3, Canada.