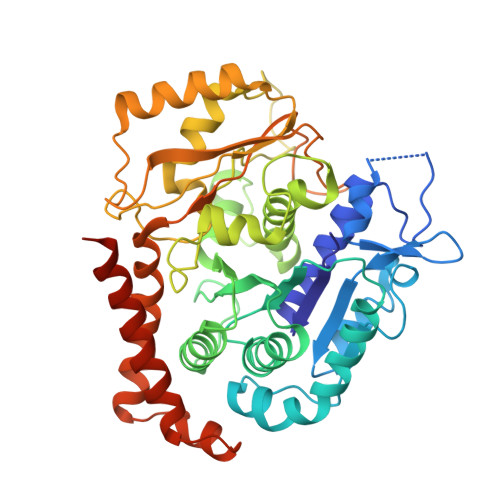
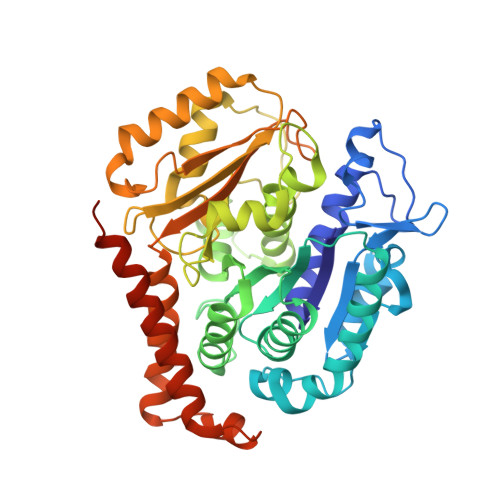
Discovery of cytotoxic dolastatin 10 analogues with N-terminal modifications.
Maderna, A., Doroski, M., Subramanyam, C., Porte, A., Leverett, C.A., Vetelino, B.C., Chen, Z., Risley, H., Parris, K., Pandit, J., Varghese, A.H., Shanker, S., Song, C., Sukuru, S.C., Farley, K.A., Wagenaar, M.M., Shapiro, M.J., Musto, S., Lam, M.H., Loganzo, F., O'Donnell, C.J.(2014) J Med Chem 57: 10527-10543
- PubMed: 25431858
- DOI: https://doi.org/10.1021/jm501649k
- Primary Citation of Related Structures:
4X1I, 4X1K, 4X1Y, 4X20 - PubMed Abstract:
Auristatins, synthetic analogues of the antineoplastic natural product Dolastatin 10, are ultrapotent cytotoxic microtubule inhibitors that are clinically used as payloads in antibody-drug conjugates (ADCs). The design and synthesis of several new auristatin analogues with N-terminal modifications that include amino acids with ¦Á,¦Á-disubstituted carbon atoms are described, including the discovery of our lead auristatin, PF-06380101. This modification of the peptide structure is unprecedented and led to analogues with excellent potencies in tumor cell proliferation assays and differential ADME properties when compared to other synthetic auristatin analogues that are used in the preparation of ADCs. In addition, auristatin cocrystal structures with tubulin are being presented that allow for the detailed examination of their binding modes. A surprising finding is that all analyzed analogues have a cis-configuration at the Val-Dil amide bond in their functionally relevant tubulin bound state, whereas in solution this bond is exclusively in the trans-configuration. This remarkable observation shines light onto the preferred binding mode of auristatins and serves as a valuable tool for structure-based drug design.
Organizational Affiliation:
Worldwide Medicinal Chemistry, Oncology, Pfizer Worldwide Research and Development , Eastern Point Road, Groton, Connecticut 06340, United States.