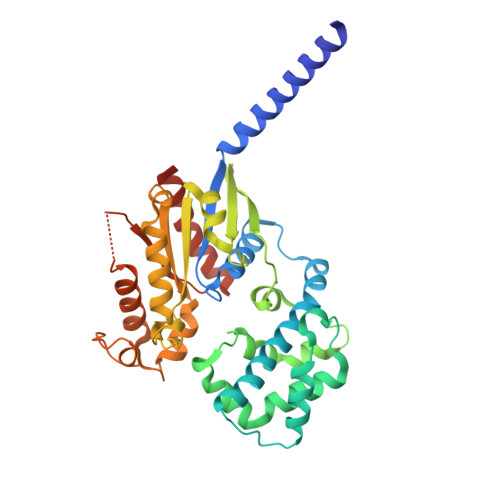
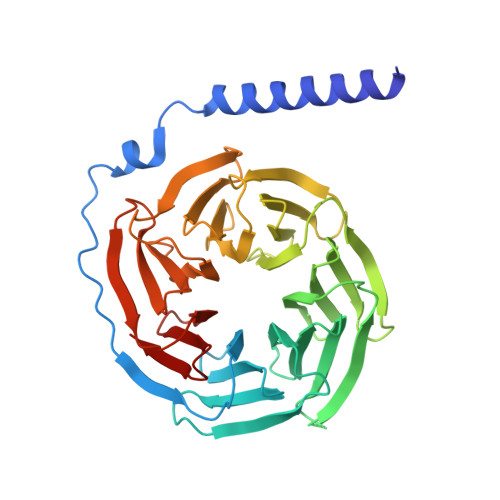
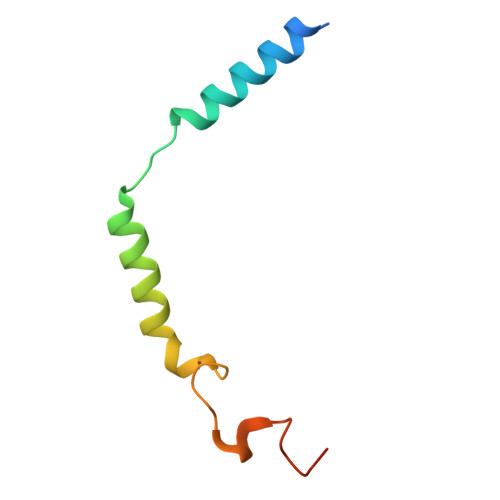
Cyclic peptide inhibitors function as molecular glues to stabilize Gq/11 heterotrimers.
Muhle, J., Alenfelder, J., Rodrigues, M.J., Jurgenliemke, L., Guixa-Gonzalez, R., Gratz, L., Andres, F., Bacchin, A., Hennig, M., Schihada, H., Crusemann, M., Konig, G.M., Schertler, G., Kostenis, E., Deupi, X.(2025) Proc Natl Acad Sci U S A 122: e2418398122-e2418398122
- PubMed: 40333756
- DOI: https://doi.org/10.1073/pnas.2418398122
- Primary Citation of Related Structures:
8QEG, 8QEH - PubMed Abstract:
Heterotrimeric G¦Á:G¦Â¦Ã G proteins function as molecular switches downstream of G protein-coupled receptors (GPCRs). They alternate between a heterotrimeric GDP-bound OFF-state and a GTP-bound ON-state in which G¦Á GTP is separated from the G¦Â¦Ã dimer. Consequently, pharmacological tools to securely prevent the OFF-ON transition are of utmost importance to investigate their molecular switch function, specific contribution to GPCR signal transduction, and potential as drug targets. FR900359 (FR) and YM-254890 (YM), two natural cyclic peptides and highly specific inhibitors of Gq/11 heterotrimers, are exactly such tools. To date, their efficient and long-lasting inhibition of Gq/11 signaling has been attributed solely to a wedge-like binding to G¦Á, thereby preventing separation of the GTPase and ¦Á-helical domains and thus GDP release. Here, we use X-ray crystallography, biochemical and signaling assays, and BRET-based biosensors to show that FR and YM also function as stabilizers of the G¦Á:G¦Â¦Ã subunit interface. Our high-resolution structures reveal a network of residues in G¦Á and two highly conserved amino acids in G¦Â that are targeted by FR and YM to glue the G¦Â¦Ã complex to the inactive G¦Á GDP subunit. Unlike all previously developed nucleotide-state specific inhibitors that sequester G¦Á in its OFF-state but compete with G¦Â¦Ã, FR and YM actively promote the inhibitory occlusion of G¦Á GDP by G¦Â¦Ã. In doing so, they securely lock the entire heterotrimer, not just G¦Á, in its inactive state. Our results identify FR and YM as molecular glues for G¦Á and G¦Â¦Ã that combine simultaneous binding to both subunits with inhibition of G protein signaling.
Organizational Affiliation:
Laboratory of Biomolecular Research, PSI Center for Life Sciences, Villigen 5232, Switzerland.